- Beauty
Human beings cannot survive without a supply of energy. What we normally use is usually only the energy released by chemical reactions, which, from a microscopic point of view, only involve the outer electrons of an atom, while the more powerful energy is hidden in the nucleus of an atom, also known as nuclear energy (also known as atomic energy).
There are two main ways for humans to obtain nuclear energy, namely nuclear fusion and nuclear fission, which simply means "Squeezing" Together a number of light atomic nuclei, and "Breaking apart" Heavy atomic nuclei.
To date, hundreds of fission-based nuclear power plants have been built, although the nuclear fuel used in these plants is uranium and plutonium.

So the question arises, with so many heavy elements on the planet, why can only uranium and plutonium be used as nuclear fuel? But not the other elements? Let's talk about this topic below.
The nucleus of an atom is made up of protons and neutrons, and in the case of antimatter, antiprotons and antineutrons, which are collectively known as "Nucleons". However, scientists have found that the average mass of the nuclei of different elements is different.
Of all the known elements, iron nuclei have the lowest average mass of "Nucleons", while the average mass of "Nucleons" Of the other elements gradually increases as the atomic number increases (larger than iron) or decreases (smaller than iron).
This means that a nucleus with a lower atomic number than iron undergoes fusion, or a nucleus with a higher atomic number than iron undergoes fission, both of which result in a loss of mass, which is then converted into energy, as described by einstein's mass-energy equation "E=mc^2".

Although theoretically, any nucleus with a larger atomic number than iron can release energy through nuclear fission, due to the limitations of technology, mankind cannot manipulate matter in terms of the size of the nucleus as it wishes, so mankind can only choose the least difficult element for nuclear fuel, which probably requires the following conditions to be met.
The larger the nucleus, the less stable it is, and the more likely it is to undergo nuclear fission and release more energy.
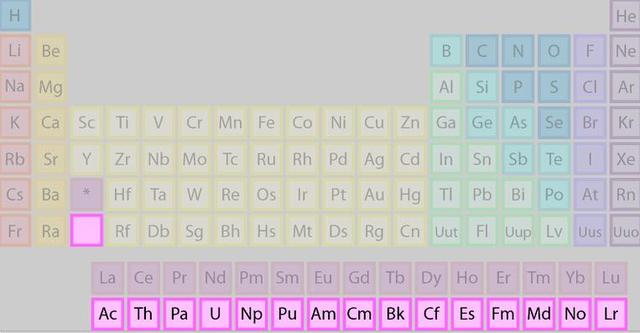
As you can see, this condition alone eliminates many "Candidates" For nuclear fuel.
- The ability to be stable over time: The actinides are all radioactive elements that decay spontaneously, and it is clear that elements with too short a half-life are not suitable for use as nuclear fuel.
- Capable of self-sustaining chain fission: It is not practical to 'break open' the nuclei one by one, so it would be better if we could just provide an initiation step, and then the nuclear fuel could continue to fuse and release energy without the need for external energy.
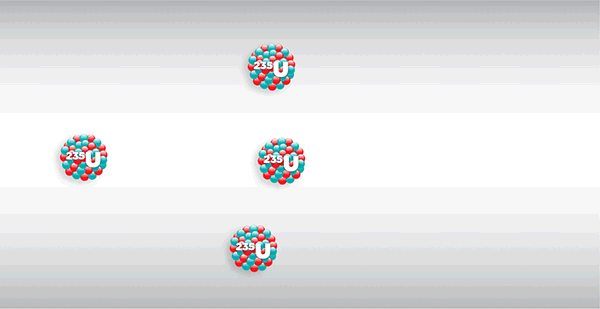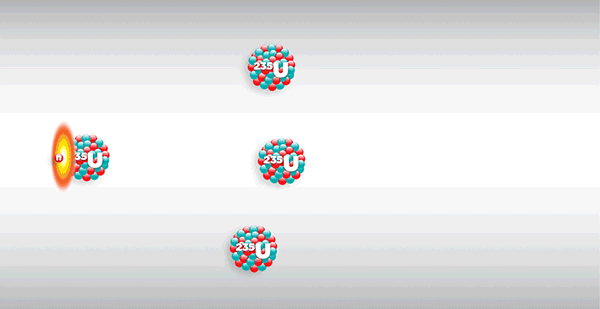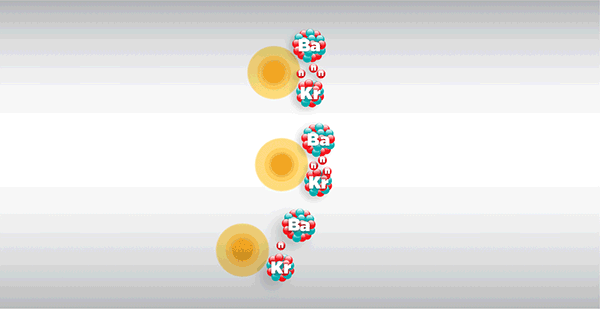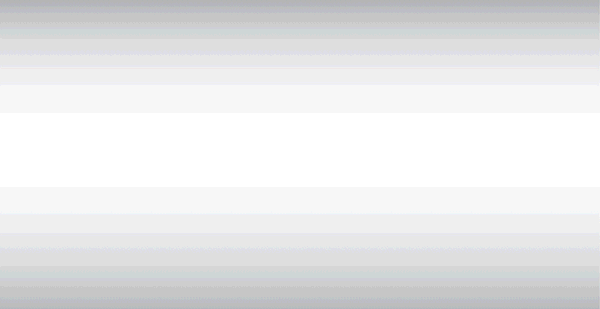
As shown in the diagram above, the fission of a nucleus releases several neutrons which, if sufficiently energetic, may trigger the fission of other nuclei, known as 'self-sustaining chain fission'.
- The fission process of a nuclear fuel must be controlled, otherwise ......
Of all the known elements, only uranium-235 and plutonium-239 meet all of these conditions, which is why uranium and plutonium are the only nuclear fuels currently used by mankind.
To add to this, although there is very little plutonium-239 in nature, plutonium-239 can be synthesised in large quantities by artificial nuclear reactions.
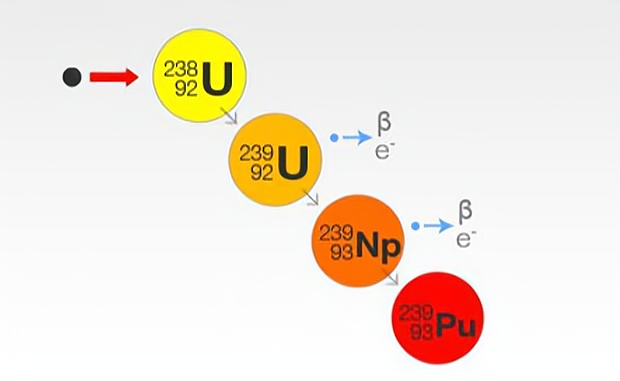
It is not that other elements on earth cannot be used for nuclear fuel, it is just that mankind has not yet mastered the appropriate technology for the time being. It is conceivable, however, that as human technology continues to advance, nuclear fuel will not be limited to a few specific elements, and perhaps in the not too distant future, humans may even be able to make nuclear fuel directly from rocks that can be found everywhere.