- Beauty
Life is a miracle in the universe and so far we have never found any identifiable trace of life outside the earth.

Studies have shown that water was present on earth in abundance more than four billion years ago. So the question arises: Has the earth's water been used for more than 4 billion years and has it become less? The scientific community is also very interested in this question, and in fact, in past research work, scientists have found the answer on a rock - what exactly is going on?
The first thing to say is that the earth is not a completely closed system. Apart from that, the earth's gravity is not as strong as it is thought to be. In the earth's atmosphere, although the earth can bind heavier elements like oxygen and nitrogen and water molecules by virtue of its gravity, for very light elements like hydrogen and helium in the atmosphere, the earth's gravity cannot form effective bindings on them.
We all know that water molecules are actually made up of hydrogen and oxygen bonded together covalently, so if there was some mechanism to break them down into hydrogen and oxygen, it is likely that the hydrogen would escape and the oxygen would remain, and if this happened, it would mean that there would be less water on earth.
Such a mechanism exists on earth, for example, short-wave radiation from sunlight can break down the covalent bonds within water molecules and break them down into hydrogen and oxygen, which is also known as 'photolysis'.
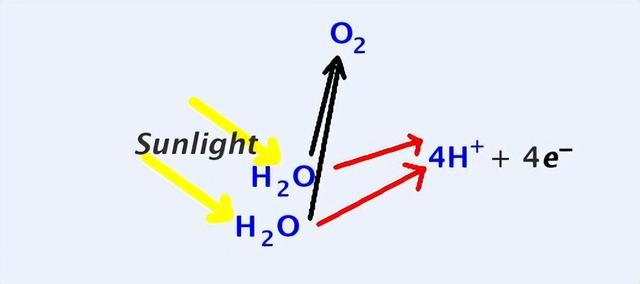
In fact, when water vapour in the earth's atmosphere diffuses into the upper atmosphere, it can be 'photolysed' by sunlight into hydrogen and oxygen, after which the hydrogen may rise due to its low density and eventually escape from the top of the atmosphere.
It is important to note that most of the water vapour in the atmosphere does not diffuse into the upper atmosphere due to the earth's gravity, and scientists calculate that only about 95,000 tonnes of hydrogen escapes from the top of the earth's atmosphere each year.
This amount is negligible compared to the amount of water on the earth, which means that the loss of water on the earth due to this cause is not significant. This is enough to compensate for the loss of water due to "Photolysis", so the earth can still "Break even" Dynamically.
For example, when seawater from the oceans seeps deep underground through cracks in the rocks of the ocean floor, it can react with hot magma and the crystalline bedrock within it, breaking down water molecules into hydrogen and oxygen, a process scientists call 'methanogenesis', as shown in the diagram below. The diagram shows this.
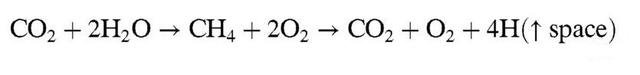
It is important to note that the diagram above shows the net effect of the 'methanogenic process', which requires specialist geochemical knowledge, and we will not expand on this here. In simple terms, this series of reactions breaks down some of the water molecules in seawater into hydrogen and oxygen, which rise to the surface of the ocean and then enter the earth's atmosphere.
The modern earth's atmosphere is so rich in oxygen that the vast majority of this hydrogen is re-oxidised into water and does not escape from the earth's top atmosphere, but the earth's atmosphere was not always as full of oxygen as it is today. In fact, before the great oxidation event some 2.6 billion years ago, the earth's atmosphere was so low in oxygen that it was negligible.
This means that before there was enough oxygen in the earth's atmosphere, the hydrogen produced by the 'methanogenic process' would have escaped from the earth's top atmosphere in large quantities, and the earth would have had less water as a result.
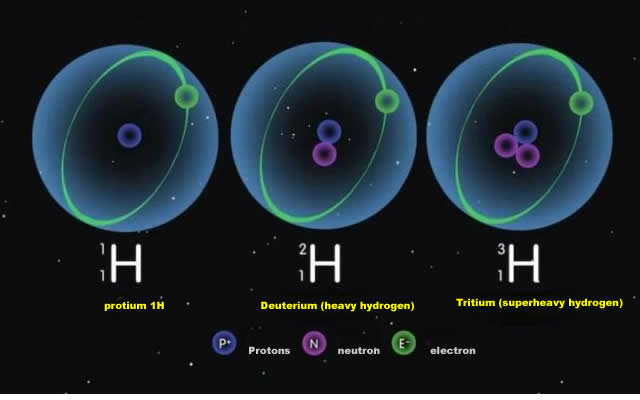
There are three isotopes of hydrogen, and the only stable ones are "Protium" And "Deuterium", which scientists have found to be more efficient than "Protium" In the "Methanogenic process" Described above. Protium" Is much more efficient than "Deuterium" In the above mentioned "Methanogenic process", which means that more "Protium" Than "Deuterium" Is in the hydrogen that escapes through the "Methanogenic process". "As the process continues, the ratio of protium to deuterium in the ocean will change.
On this basis, we simply need to know the ratio of protium to deuterium in the earth's oceans more than four billion years ago, use the relevant theory to build a model, and compare it with the ratio of protium to deuterium in the modern earth's oceans. "This will allow us to calculate how much hydrogen the earth has lost in the past, and how much water the earth has lost in the past (after all, most of the water on earth is in the oceans).
I am sure you have already guessed what "Scientists found the answer on a rock" Means. The ratio of "Deuterium" To "Protium" In the oceans of ancient earth was found on a rock.

This stone was found in a geological formation in western greenland and has been determined to be a serpentinite formed about 4 billion years ago. The ratio of "Protium" To "Deuterium" Is preserved for a long time.
From studying this stone, scientists have concluded that the modern earth's oceans are about 26% smaller in volume compared to the early earth, in other words, there is almost a quarter less water on the planet.

(the picture shows this rock under a microscope)
This is a surprising answer, but we needn't worry that water on the modern earth will continue to get substantially less.
As mentioned above, the modern earth's atmosphere is already full of oxygen, and this means that on the modern earth, the hydrogen produced by the 'methanogenic process' is largely oxidised to water in the lower atmosphere, and does not escape from the top of the earth's atmosphere.
Even if a very small amount escapes, and the water on earth is thus reduced a little, this is much less than the water lost on earth through 'photolysis', and can be replenished by free hydrogen in space and by small water-rich bodies, thus maintaining a dynamic equilibrium overall.






